
 Web Content Display
Web Content Display
SOLARIS centre
 Web Content Display
Web Content Display
 Web Content Display
Web Content Display
Understanding photosynthesis at atomic resolution
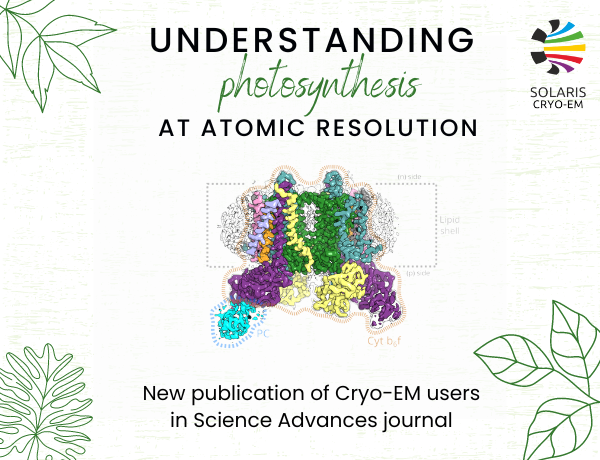
SOLARIS users have studied photosynthetic processes at the atomic level. The result was obtaining high-resolution cryo-EM structures of the cytochrome b6f protein complex, one of the key proteins of photosynthesis.
Cytochrome b6f
Scientists from the Faculty of Biochemistry, Biophysics and Biotechnology (FBBB) and the Małopolska Centre of Biotechology (MCB) of the Jagiellonian University Krakow teamed up to understand photosynthesis in plants at the atomic level. In detail, the Department of Molecular Biophysics and the Max Planck Research Group determined high-resolution cryo-EM structures of cytochrome b6f, one of the key membrane protein complex in photosynthesis. This complex transfers electrons between plastoquinol and plastocyanin to link functionally plant photosystems, thereby securing electron flow and efficiency of photosynthesis.
The seminal study was published in a recent issue of Science Advances.
The complex and the structures
The first structure (at 2.7 Å resolution) reveals how plastocyanin binds to dimeric cytochrome b6f complex to efficiently accept electron released during the catalytic reaction. It unexpectedly shows only one plastocyanin in the dimer. The second structure (at a remarkable 2.1 Å resolution) identifies a chain of plastoquinone molecules lining up one after another in a manner implicating the existence of a channel in each monomer with spatially distinct entrance and exit points. This suggests an entirely new and unanticipated traffic of quinones in which they flow through the channel in one direction, transiently passing through the catalytic site. The proposed mechanism differs from the previously considered reaction scheme, where the substrate and product were assumed to use the exact same channel to enter and leave the catalytic site. In addition, the structure revealed the presence of an unexpected cytochrome b6f binding partner, namely thylakoid soluble phosphoprotein TSP9 (protein restricted to higher plants), bound in the highly specific manner at the site presumed to be important for controlling the photosynthetic electron flow. The first author Marcin Sarewicz (Department of Molecular Biophysics, FBBB) explains – “Our discovery provides new perspective on understanding the mechanism of regulation of photosynthesis at the level of cytochrome b6f with participation of TSP9 protein.”
Fig.1. Structure of the Cyt b6f–PC complex.
The significance of the discovery
Artur Osyczka (Department of Molecular Biophysics, FBBB) summarizes – “Overall, it is expected that new structural information provided by the published structures will contribute to our better understanding of the molecular basis of the energetic efficiency of photosynthesis and will inspire further spectroscopic and kinetic explorations.” Sebastian Glatt (Max Planck Research Group, MCB) adds – “Because of the exceptionally high resolution - this is so far the best-resolved structure of cytochrome b6f from higher plants - the structures will provide useful models for quantum mechanical calculations aimed at further unraveling physico-chemical basis of key photosynthetic reactions catalyzed by cytochrome b6f.” The study also exemplifies how researchers from different research units at the Jagiellonian University can team up and efficiently work together to deliver scientific results at the world class level.
The publication is available in Science Advances:
The team first isolated and characterized biochemically the proteins from spinach leaves at FBBB and then prepared all samples for structural analysis at the MCB Structural Biology Core Facility. All data were collected at the Titan Krios G3i high-end cryo-electron microscope located at SOLARIS National Synchrotron Radiation Centre. This project was financed by Foundation for Polish Science (grants TEAM to A. Osyczka, and TEAM TECH Core Facility to S. Glatt).
