EXAFS - example 1
EXAFS spectroscopy provides information about the average coordination number around the absorber atoms and bond distances.
Figure 1. Schematic illustration of Pt atoms deposited on nitrogen-doped carbon dots (Pt–NCDs) and (b) Fourier transformed Pt L3-edge EXAFS spectra of Pt–NCDs hybridized with TiO2 film, and of PtO2, PtCl2 and Pt foil as references for comparison (measured at beamline B18, Diamond Light Source).
The absence of a scattering peak in the region 2-3.5 Å, corresponding to a Pt-Pt bond, indicates that only isolated Pt atoms are bound to the NCD support, and the single peak at 1.6 Å reveals that they are coordinated to light atoms (4-5 carbon atoms) on the support.
This example shows that EXAFS data can be used to distinguish between single-atom catalysts and small clusters or nanoparticles bound to the support. Pt single-atoms are important as catalysts for photocatalytic hydrogen production.
Adapted from:
EXAFS - example 2
EXAFS spectroscopy provides information about the average coordination number around the absorber atoms and bond distances.
Figure 2. (left) Preparation of 17 atoms Pt clusters deposited on γ-Al2O3 (Pt17/γ-Al2O3), (right) Fourier transform of Pt L3-edge EXAFS spectra of [Pt17(CO)12(PPh3)8]Cln, Pt17(CO)12(PPh3)8/γ-Al2O3, and Pt17/γ-Al2O3 together with EXAFS data of Pt foil and PtO2. In the spectrum of Pt17/γ-Al2O3 the peak at 1.7 Å is attributed to Pt–C or Pt–O bonds at the Pt17/γ-Al2O3 interface.
EXAFS data shows that in the Pt17/γ-Al2O3 system the supported Pt17 is not present in the form of oxide but has a framework structure like a metal cluster. Moreover, it was shown that supported Pt17 clusters are covered by CO molecules at normal temperature. CO molecules adsorbed on fine Pt17 supported clusters generally has a longer C–O bond compared to larger Pt supported nanoparticles, promoting the oxidation reaction and possibly contributing to the high catalytic activity of the Pt17/γ-Al2O3 system for carbon monoxide and propylene oxidation in comparison to γ-Al2O3-supported larger Pt nanoparticles (PtNP/γ-Al2O3)prepared by conventional impregnation.
Adapted from:
XANES - example 1
XANES spectra are highly sensitive to the coordination environment of the absorber atoms (e.g., oxidation state).
Figure 3. Sulphur K-edge XANES.
Sulfur K-edge XANES spectra of substances with sulphur atoms in different oxidation states, attached to different ligands and in different coordination geometries. Obviously, different coordination environments cause characteristic features in XANES spectra, which can serve as ´spectral fingerprints´, and the sulfur speciation in an unknown sample can be determined by comparison with reference XANES spectra. Moreover, XANES spectra can be fit to linear combinations of reference spectra, from which the relative concentrations of the different species can directly be obtained.
Adapted from:
XANES - example 2
XANES spectra are highly sensitive to the coordination environment of the absorber atoms (e.g., oxidation state).
Figure 4. Linear combination fitting (LCF).
Linear combination fitting (LCF) of Cl K-edge XANES spectra of Ce glass-ceramics with nominal (a) 0.9 wt% Cl and (b) 1.7 wt% Cl; (c) comparison of the weighted contributions of reference compounds fitted to the Cl K-edge XANES data of Ce-free and Ce-incorporated glass-ceramics (samples were fabricated with CeO2, which model a PuO2 surrogate). XANES spectroscopy was used to investigate the potential application of an albite glass-zirconolite ceramic material for immobilisation of chloride contaminated plutonium oxide residues. XANES data confirm partitioning of Cl to the glass phase with exsolution of crystalline NaCl above the chlorine solubility limit. LCF results indicate an association of Cl with Na and Ca modifier cations, with environments characteristic of the aluminosilicate chloride minerals eudialyte, sodalite, chlorellestadite and afghanite. This study demonstrates the compatibility of the glass-ceramic wasteform toward Cl solubility at the expected incorporation rate (below the determined solubility limit) and provides confidence that upstream heat treatment or blending of waste feed are not required.
Adapted from:
XANES - example 3
XANES spectra are highly sensitive to the coordination environment of the absorber atoms (e.g., oxidation state).
Figure 5. Vanadium K-edge XANES spectra.
Vanadium K-edge XANES spectra of (a) electrochemically lithiationed V2O3(SO4)2 electrodes at various states of discharge and charge and (b) chemically lithiated V2O3(SO4)2 samples compared to the reference materials V2O5, VOSO4·3H2O and V2O3; (c) vanadium K-edge Energy (at half-height) as a function of oxidation state. In this case, XANES spectroscopy was used to investigate Li+ insertion into V2O3(SO4)2 (promising material for high energy density lithium-ion batteries) via electrochemical and chemical routes. The results show that 2.0 Li+ ions can be incorporated electrochemically, resulting in Li2V2O3(SO4)2 attributed to the V4+/V5+ redox couple. For the chemically lithiated materials up to 4.0 Li+ ions can be inserted into V2O3(SO4)2, reducing V5+ to V3+.
Reproduced from:
Potassium hexavanadate (K2V6O16·nH2O) nanobelts have been synthesized by the LPE-IonEx method, which is dedicated to synthesis of transition metal oxide bronzes with controlled morphology and structure. The electrochemical performance of K2V6O16·nH2O as a cathode material for lithium-ion batteries has been evaluated. The KVO nanobelts demonstrated a high discharge capacity of 260 mAh g-1, and long-term cyclic stability up to 100 cycles at 1 A g-1. The effect of the vanadium valence state and unusual construction of the nanobelts, composed of crystalline and amorphous domains arranged alternately were also discussed in this work. The ex-situ measurements of discharged electrode materials by XRD, MP-AES, XAS and XPS show that during the subsequent charge/discharge cycle the potassium in the K2V6O16·nH2O structure are replacing by lithium. The structural stability of the potassium hexavandate during cycling depends on the initial vanadium valence state on the sample surface and the presence of the “fringe free” domains in the K2V6O16·nH2O nanobelts.
The electrochemical performance, as well as the structural flexibility of K2V6O16·nH2O, strongly depends on the vanadium valence state. The charge on hydrated potassium vanadate is stored via redox reaction mainly at the surface. Thus, via the presence of the V4+ on the surface, the electronic transfer is facilitated, and higher capacity is achievement. Moreover, the higher V4+ surface concertation leads to faster structural changes during subsequent charge/discharge cycles. The ex-situ characterization by XRD and MP-AES shows that during the subsequent charge/discharge cycle, the potassium ions in the K2V6O16·nH2O structure are replacing by lithium. However, the higher initial concentration of V4+ leads to increased vacancies gradually during cycling, both on the surface and in the bulk as confirmed by the ex-situ XPS and XANES analysis. Therefore, the structural damage occur slowly. The unusual construction of the nanobelts, composed of crystalline and amorphous domains arranged alternately, probably also prevents the crystal structure from collapsing during this exchange.
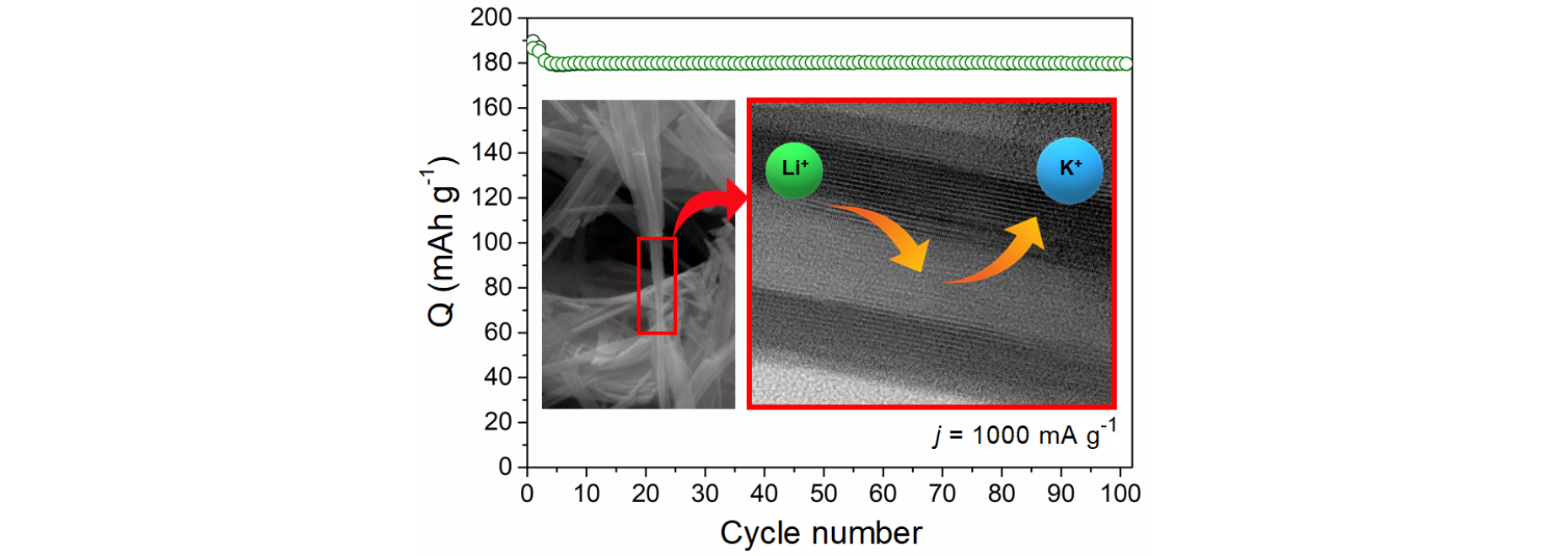
Fig.1. Graphical abstract.
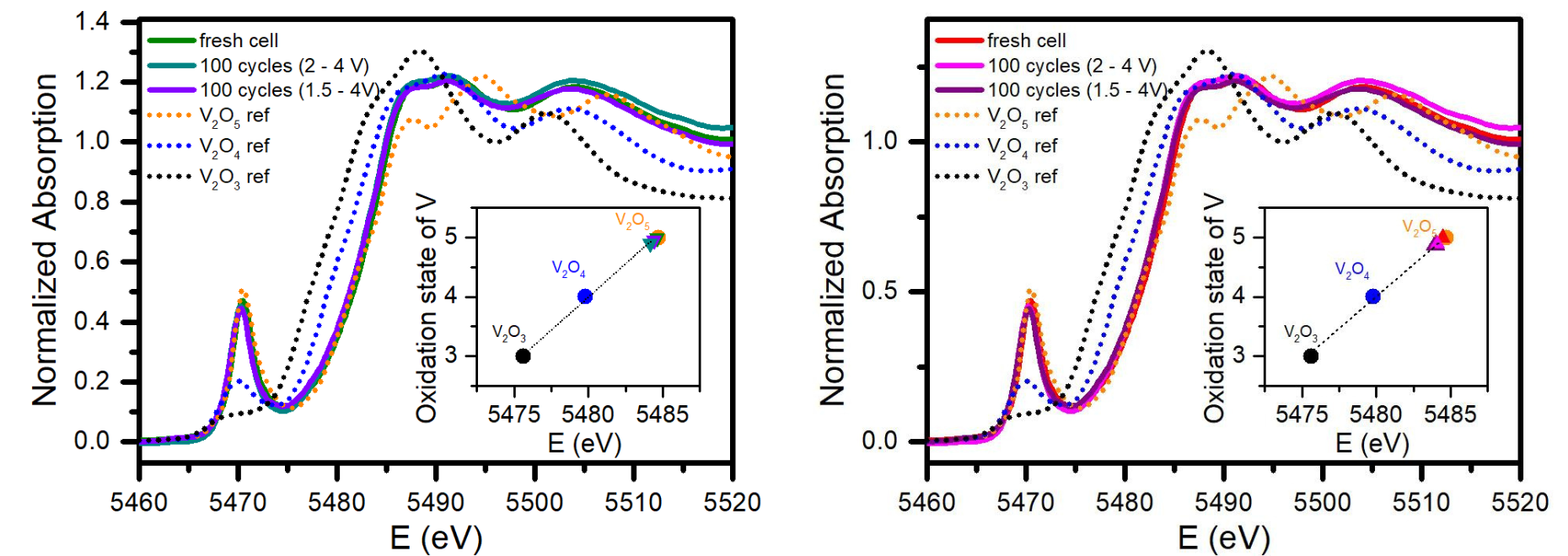
Fig.2. V K-edge the XANES for samples KVO-20 (left) and KVO-40 (right), respectively. The insets show the relation between vanadium oxidation state and the edge position.
Reproduced from:
M. Przesniak-Welenc, M. Nadolska, K. Jurak, J. Li, K. Gornicka, A. Mielewczyk-Gry, M. Rutkowska, and A. P. Nowak, The Valance State of Vanadium-Key Factor in the Flexibility of Potassium Vanadates Structure as Cathode Materials in Li-Ion Batteries, Sci Rep 12, 18751 (2022).
Beamline team develops ideas and prepositions of ASTRA beamline application for industry partners.
Until a detailed concept for the industrial use of ASTRA has been defined, we encourage you to visit the SOLARIS webpage, where main ideas of SOLARIS´s collaboration with industry partners are presented here.
EU HORIZON2020 program SYLINDA
Hochschule Niederrhein University of Applied Sciences (Germany) in collaboration with the Physics Institute University of Bonn and National Synchrotron Radiation Centre SOLARIS Jagiellonian University.
This project contributes to strengthen the capacities of research institutions of the widening countries. In particular, SYLINDA aims at boosting the research and development capabilities of SOLARIS, the Polish Synchrotron facility, based on the experience brought by the partners’ consortium: Synchrotron SOLARIS (Poland), ALBA Synchrotron (Spain), Hochschule Niederrhein University of Applied Sciences (Germany), Rheinische Friedrich-Wilhelms-Universitat Bonn (Germany).
The X-ray Absorption Spectroscopy (XAS) beamline ASTRA (XAS-HN) of SOLARIS is to be upgraded with a high-resolution spectrometer which will make it scientifically excellent and very attractive for academic and industrial users dealing with low atomic number elements studies.
New industry cooperation avenues with the pharmaceutical-, rubber-, agro-, (micro-) biological, chemical- and cosmetics sectors will be consequently opened. A focused communication plan together with an industrial workshop will put SOLARIS in the European scene of those scientific and industrial areas.
All those activities are the first step forward that will be sustained beyond the end of this project thanks to the networking and links established with the experienced partners that will open the doors to SOLARIS for future collaborations with other European and worldwide research institutions. No doubt this project aims to represent a quantum leap for SOLARIS in research and development terms.
Start: January 2021.
End: December 2023.

