Application examples
- Catalysis - a research area focusing on the development of catalysts showing greater activity or selectivity in chemical processes of technological or industrial importance. The use of model systems represents an effective approach to obtaining information on the basic properties of catalysts, which allows understanding how such materials perform under real operating conditions. This knowledge is extremely important because it allows the catalyst to be optimized both in terms of structure and chemical composition, allowing for rational design of new materials. The use of LEEM / PEEM methods for modeling systems in catalysis is very important due to many aspects. First of all, these techniques allow you to conduct experiments and track many different processes in real time, in situ and operando. One of the main advantages of both LEEM and PEEM is the ability to track structural and chemical changes on the surface of model catalysts in real time. and under strictly defined conditions under which chemical reactions take place. Additionally, the PEEM microscope allows the observation of reactions with spatial resolution.
- Magnetism - this is another research area that addresses the engineering of new materials. In particular, the study of the static and dynamic properties of magnetic domains in ferromagnetic nanostructures is of great interest due to the technological possibilities based on the movement of domain walls (DW) and the possibility of using the dynamics of these processes in magnetic memories. Access to synchrotron radiation with variable polarization and the ability to work in the XMCD / XMLD PEEM mode (circular and linear dichroism) allows for the characterization of magnetic properties in the nano scale, which is not covered by other devices. It is also possible to observe changes in magnetic structures in real time as a result of the application of additional factors during imaging, e.g. an external magnetic field or an electric field. These ferromagnetic nanostructures constituted the group of materials that was most often examined by users of the PEEM microscope in the SOLARIS synchrotron.
- 2D materials - Advances in research into two-dimensional (2D) materials have opened new possibilities for the miniaturization of optoelectronic and spintronic devices at atomic scales. One of the main areas of research that is the subject of intensive work is the surface and electronic structure of 2D materials and their van der Wasal (VdW) heterostructures, which may be significantly influenced by the local geometry of atoms and their local chemical and electronic environment. In 2D material research, advanced microscopy and spectroscopy techniques must be used to characterize material properties on a micro- or sub-micrometer scale. Spectroscopic photoemission combined with low energy electron microscopy available on the DEMETER beamline (SPE-LEEM) offers a multifunctional approach that uniquely integrates microscopy, diffraction and spectroscopy techniques, enables real-time imaging, and provides structural, chemical and electronic analysis of surfaces and interfaces in situ. In addition to the highlighted capabilities of the PEEM microscope, the station also offers measurements in the X-ray angle-resolved photoelectron spectroscopy (ARPES) mode. This method is necessary to study the band structure of materials and to determine the electronic properties. Measurements allow to determine the correlation between the structural, chemical and electronic properties as well as the chemical composition of the tested material.
To study the synergy between the transition metals for enhancing the electrochemical and chemical activity, a series of SOECs were modified with a small amount of Co ions, namely 1.8, 3.6, and 5.4 wt% in the reduced state. Full characterization involving STXM imaging allowed for better understanding of the synergy between the Ni and Co host metal and made it possible to find the causes of the increased activity. It revealed the complexity of the substructures formed within the electrode. The performance tests indicated the roles of both rWGS and direct electrolysis of CO2 in the electroreduction process.
The modifications on SOECs consisted of the introduction of small amounts of Co into the Ni-YSZ cermet material of the cells via the wet impregnation method. Thanks to the STXM imaging coupled with XAS measurements it was observed that the Co ions formed three types of substructures, namely nanoparticles of CoxOy supported on the surface of the 8YSZ, Ni-Co mixed spinel-like compound on the interface between the Ni core and outer layer, and CoxOy nanoparticles embedded into the spinel scale (Fig. 1.). The XPS results indicated that Co induced the formation of a high amount of the available catalytic sites through the active Ni3+/Ni2+ and Co3+/Co2+ couples for reactions to happen. The performed modifications increased the CH4 concentration in the outlet stream over 2.5 times and ensured better efficiency of the H2O/CO2 co-electrolysis (Fig. 2). The search for the possible causes of the enhancement through XAS and STXM measurements resulted in direct proof of the existence of a significant amount of the intermixed Ni-Co compound which induced changes in the shift of the EF band energy, generated the inverse spinel structure, and introduced a significant amount of active surface species. The STXM measurements clearly evidenced a Co-graduated structure of core-shell-like Ni grains. The addition of the secondary metal into the Ni-YSZ conventional cermet material revealed highly promising results to be further applied in the field of H2O/CO2 co-electrolysis with the simultaneous single-process of methanation for the buildup of advanced conversion systems.

Fig.1. STXM images of the as-prepared 3.6 wt % Co-impregnated sample with the corresponding elemental distribution maps of Ni and Co. The Ni and Co map overlay is also presented.
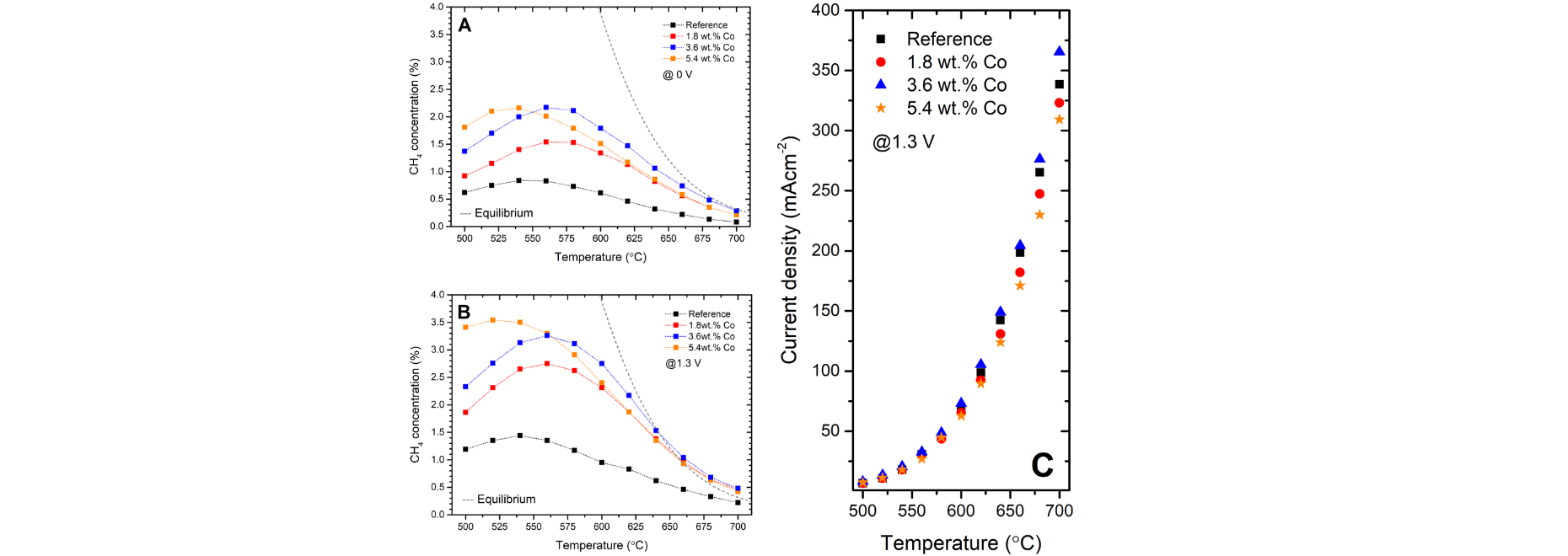
Fig.2. Concentration of CH4 in the outlet stream at OCV (A) and 1.3 V (B). Equilibrium concentration marked as dashed line. (C) Current densities at 1.3 V vs. temperature. Dashed lines are visual guides only.
Full publication available:
A novel one-pot synthesis route leading to the formation of a wormhole-like structure was developed for the successful fabrication of porous YSZ and Ni-YSZ systems. This method involved co-precipitation in the presence of CTAB/Pluronic P123 and crystallizing NaCl. The fabricated Ni-YSZ showed better long-term electrical stability in hydrogen than a traditional Ni-YSZ cermet. It resulted from the suppression of Ni structural changes throughout the anode scaffold. Therefore, the novel soft-hard templating method is recognized as a promising route for the fabrication of the YSZ or Ni-YSZ with a highly developed microstructure and improved stability.
It was proven via STXM imaging that after the precipitation, the gel was supported on a soft micelle-based template to be further structured via leftover NaCl formed during the drying step (hard template). The co-existence of those two templates with the fully amorphous and extremely nanometric precipitate of separated metal hydroxides resulted in obtaining a wormhole-like structure of either YSZ or NiO-YSZ, depending on the starting cation composition (Fig. 1.). Good separation of both composite’s subphases was evidenced despite the formation of the nanoprecipitates. It was evidenced that the concentration of 0.3 M cations in the starting solution gave the most homogeneous structure with the highest porosity of around 50% and 47% for 8YSZ and NiO-YSZ (reduced), respectively, even though no additional pore formers were added. The reduction studies proved that the novel way to prepare NiO-YSZ ensures a higher degree of integration of the NiO and YSZ grains and limits the growth of Ni grains during redox cycles. The long-run electrical conductivity test revealed that 0.3 M CTAB/NaCl NiO-YSZ is more stable within 160 h of testing than the corresponding reference material prepared using conventional methods. It was found that the restructured material is less prone to Ni agglomeration and migration across the anode, mostly due to the increased NiO-YSZ interfacial integration as well as uniform and finely dispersed Ni grains after reduction (Fig. 2).

Fig.1. STXM images of the 0.3 M CTAB/NaCl NiO-YSZ precursor powder measured at 848 eV (before the Ni edge), 853 eV (Ni edge), Ni elemental map and SEM image. Every scale bar is 1 µm.
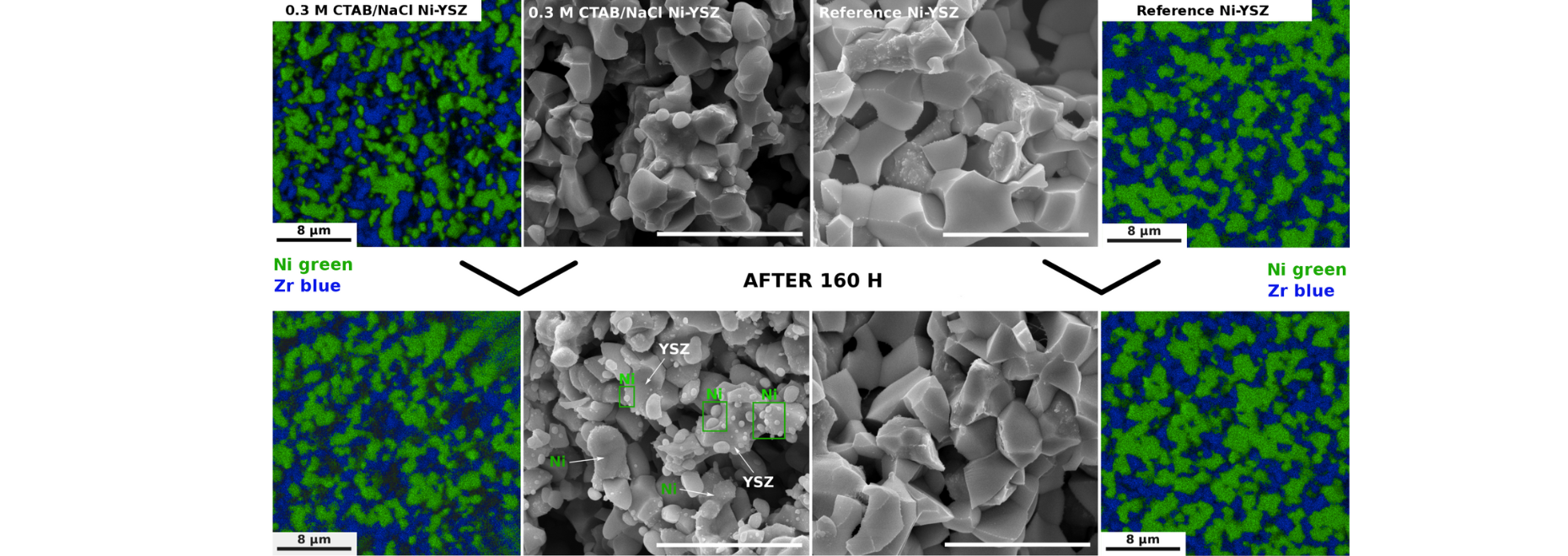
Fig.2. SEM images and EDS elemental maps of the 0.3 M CTAB/NaCl Ni-YSZ (reduced) and conventional reference anodes before and after the ageing tests. Scale bar is 8 µm each time.
Full publication available:

